
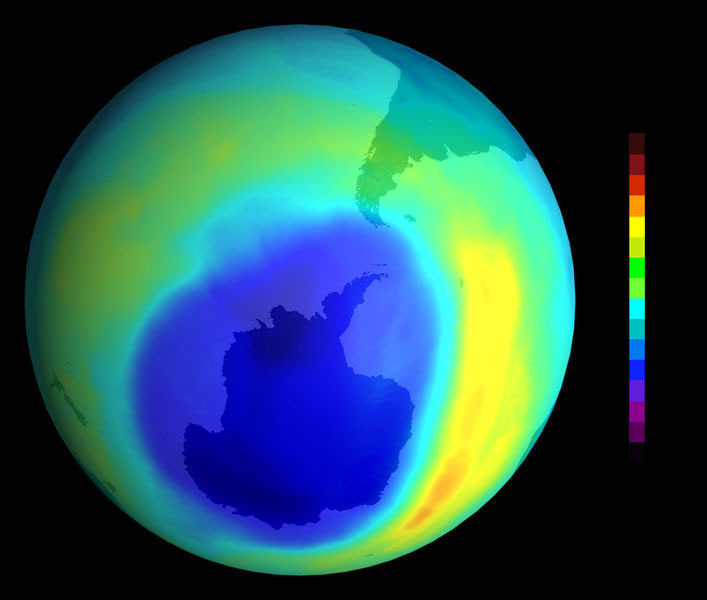
The balance of ozone and sun
Source
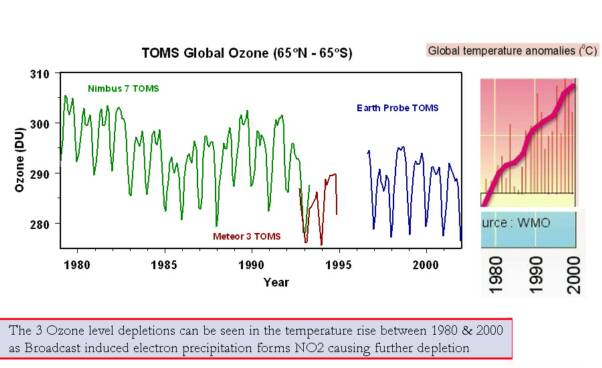
3 Ozone depletion levels
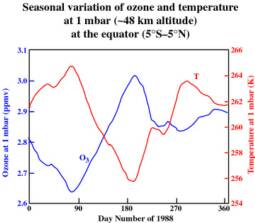
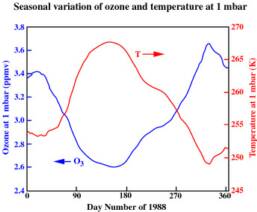
The 3 levels of global stratospheric ozone depletion are clearly visible in the global temperature showing a connection between the two. There are seasonal and solar variations in temperature that are depend on the altitude of the stratosphere and the latitude.
Learn more Ozone
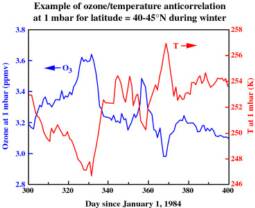
The balance of ozone and sun
The ozone layer is our most important defence against the suns powerful radiation that heats the planet. It is a fragile molecule and is easily bullied around by other chemical reactions and electrical fluctuations in the atmosphere.The highest levels of ozone in the atmosphere are in the stratosphere, in a region also known as the ozone layer between about 10 km and 50 km above earths surface. It is created by the suns UV radiation and destroyed by the reaction with atomic oxygen:
O3 + O → 2 O2
The latter reaction is catalysed by the presence of certain free radicals, of which the most important are hydroxyl (OH), nitric oxide (NO) and atomic chlorine (Cl) and bromine (Br). In recent decades the amount of ozone in the stratosphere has been declining due to three major factors. One is the emissions of CFCs and similar chlorinated and brominated organic molecules, which have increased the concentration of ozone-depleting catalysts above the natural background. The other two come in the form of particle showers that flow from the blanket of electricity that surrounds the planet called the ionosphere and the blanket of gravity called the magnetosphere. Protons come from the sun in what are known as solar proton events and can slash ozone levels derastically while the electrons come from PLHR (Power Line Harmonic Radiation) interacting with the magnetosphere and broadcast radiation from the radio, television, and telecom industry reacts with the ionosphere. This particle precipitation reacts with the chemicals in the upper atmosphere and form NO2 and OH which are carried down to the lower atmosphere by what is called the polar vortex.

T H E M I S
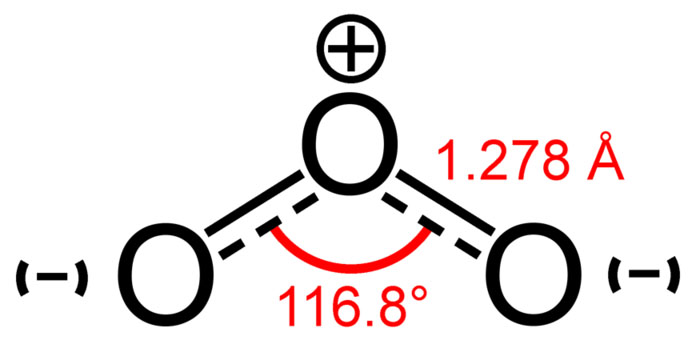
Ozone-oxygen cycle
Click to go to
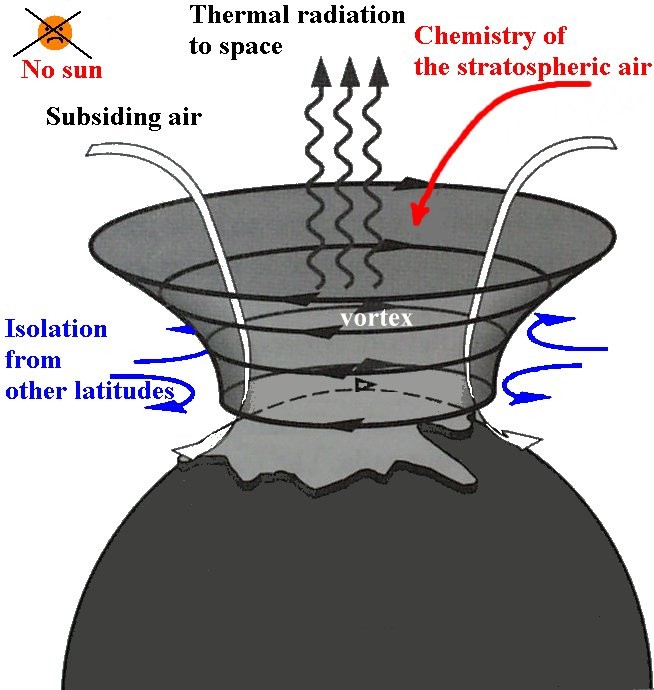
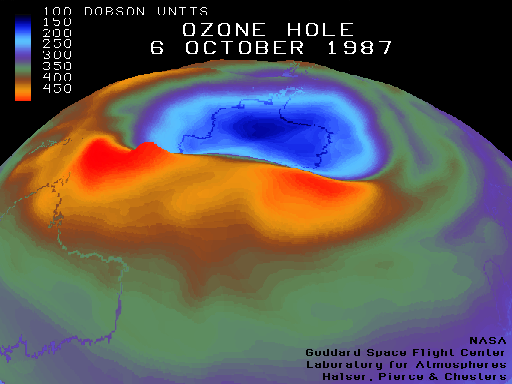

Why is the Ozone hole still growing?
How does this effect Polar ice caps?
Click to go to
Click here to Enlarge
Arctic Ozone Depletion Linked to Longevity of Polar Stratospheric Clouds - Click & go
Global Warming Theories
What causes it ?

